CureMetrix gets FDA clearance for mammogram-screening system
Mar 20, 2019 -
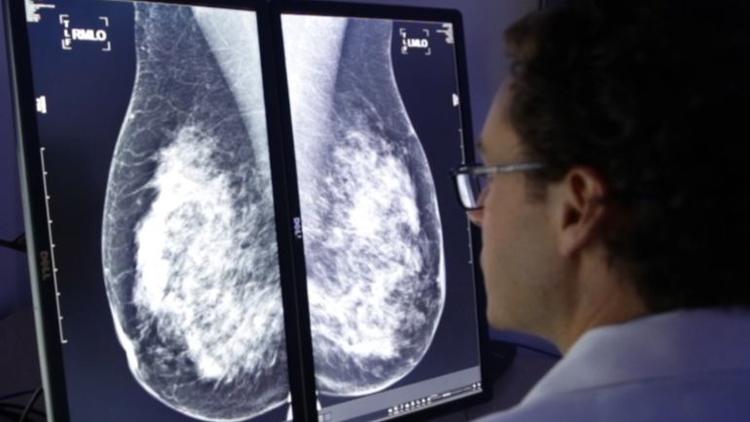
This week, SD-based biotech company CureMetrix announced that it received FDA clearance to sell its software that screens mammograms 40 percent faster than traditional methods. This could have a huge impact on clinical efficiency. Learn more by reading The San Diego Union Tribune article below:
La Jolla’s CureMetrix said Tuesday it has received FDA approval to sell its software “triage” service to screen mammograms for signs of cancer. CureMetrix’s cmTriage flags suspect mammograms so radiologists can give them a closer look, said Kevin Harris, CEO of the privately held company.
The automated process enables radiologists to devote more of their attention to abnormal mammograms instead of having to look at every image to find them. The cloud-based service sends back the results in three to four minutes, Harris said.
“What we’re seeing in preliminary studies is the triage software can help doctors read through their work list up to 40 percent faster, which as you might expect, is a big impact on the clinical efficiency,” Harris said.
Patient identifying information is removed at the hospital by the service, which is designed to work with all mammography systems, he said.
The company is right now “turning on the sales engine and working to get this out there,” he said. The cost is expected to be in the range of $1 to $5 per mammogram.
CureMetrix says radiologists can elect to get immediate notification of suspicious results, so patients can be notified before they’ve left the clinic.
Harris said radiologists can also adjust the software’s sensitivity according to their preference. A higher sensitivity catches more potential cancers, at the price of more false positive results.
The software has a 0.95 “area under the curve” ratio, a measure of accuracy, according to a CureMetrix statement. A perfect screen has an AUC of 1.0; while an AUC of 0.5 means the test operates at chance level.
Keeping false positives down while maintaining accuracy is important, the company says, because out of every 1,000 tests, only 5 find cancers at screening.
CureMetrix is also developing another software product called cmAssist that marks any suspicious regions. It also ranks the degree of abnormality to guide radiologists. That product has yet to receive FDA clearance.
CureMetrix’s website is http://curemetrix.com.
Photo by SDUT | CureMetrix.


